Abstract
Introduction: Cytogenetic analysis is important for stratifying patients with various myeloid neoplasms. It has been reported that whole-genome sequencing can be used as an alternative to cytogenetic analysis in acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS). With the increasing use of liquid biopsy in the diagnosis and monitoring of patients with various types of neoplasms, we explored the potential of using liquid biopsy and next generation sequencing (NGS) in detecting chromosomal structural abnormalities or copy number variation (CNV) in patients with myeloid neoplasms. For practical approach and for capturing single nucleotide variants (SNV) and to achieve enough depth in sequencing, we used targeted sequencing for determining the chromosomal structural abnormalities in cell-free DNA (cfDNA) in patients with myeloid neoplasms.
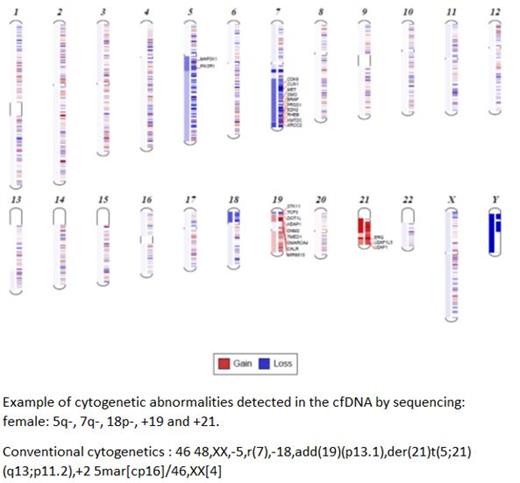
Methods: Peripheral blood plasma samples from 144 patients with myeloid neoplasms were used to extract cfDNA for NGS testing. This included 49 patients with MDS, 31 with AML, and 64 patients with myeloproliferative neoplasms (MPN). The median age was 68.5 (range: 24-96); 56 (39%) were female. cfDNA was sequenced using 275 gene panel. The panel uses single primer extension (SPE) approach with UMI. Sequencing depth was increased to more than 1000X (after removing duplicates). CNVkit software was used for analyzing and visualizing copy number variations. All samples were confirmed to be diagnostic by showing mutations in diagnostic genes with variant allele frequency >20% or by showing diagnostic chromosomal structural abnormalities (e.g., 5q deletion in MDS, 5q- syndrome). Cytogenetic data on 35 corresponding bone marrow samples (18 AML and 17 MDS) were available for comparison.
Results: Of the 144 samples, 47 (33%) showed chromosomal structural abnormalities. In the AML group, 20 of 31 (65%) showed cytogenetic abnormalities by cfDNA testing. Of these positive AML patients, 18 (90%) (58% of total AML) had poor-risk cytogenetics. Therefore, the AML patients with normal cytogenetics or cytogenetic abnormalities other than high-risk constituted 42% of total AML patients. Of the MDS group, 11 of 49 MDS patients (22%) showed cytogenetic abnormalities by cfDNA testing, 6 of whom (54.5%) had high-risk cytogenetics. Overall, 12% of all MDS had poor-risk cytogenetics by cfDNA testing. In the MPN group, 16 of 64 (25%) showed cytogenetic abnormalities, 2 of which (12.5%) had 7q deletion (3% of all MPN); the rest (87.5%) of cytogenetic-positive MPN (22% of total MPN) had other abnormalities including 20q-, +8, 12q, 17p-, 11q-, trisomy 9, trisomy 21 and others. To compare chromosomal abnormalities as detected by cfDNA NGS testing with conventional cytogenetic analysis of corresponding bone marrow samples, we classified cytogenetic findings based on risk stratification into either intermediate-risk or poor-risk. Of the 36 cases, there was 100% concordance between cfDNA data and cytogenetic data when findings were grouped based on risk classification. Two of the conventional cytogenetic samples showed no metaphases while one showed intermediate-risk abnormalities by cfDNA NGS analysis and the second showed poor-risk cytogenetic abnormalities by cfDNA NGS analysis. These 36 cases included 16 cases with normal cytogenetics. Simple abnormalities such as 5q-, 7q-, +8 were called in identical fashion, but some other abnormalities such as derivative chromosome and marker chromosome were resolved or interpreted differently by the cfDNA NGS analysis. The NGS panel design used in this study does not cover fusion genes or chromosomal translocation, and chromosomal translocations were missed at this time.
Conclusions: This data shows that liquid biopsy using and targeted NGS is reliable in detecting chromosomal structural abnormalities in myeloid neoplasms and potentially can replace the need for conventional cytogenetic testing. While the current study was not designed to detect chromosomal translocations, a small, targeted panel of 275 genes is adequate for standard risk classification of myeloid neoplasms into intermediate or high-risk. Considering that in the same test complete mutation profiling can also be achieved along with chromosomal structural analysis, liquid biopsy in myeloid neoplasms might be considered as an efficient replacement to bone marrow biopsy in patients with myeloid neoplasms when fusion genes are not expected.
Goy: Kite Pharma: Membership on an entity's Board of Directors or advisory committees; Infinity/Verastem: Research Funding; COTA (Cancer Outcome Tracking Analysis): Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Other: Leadership role; OncLive Peer Exchange: Honoraria; Bristol Meyers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Vincerx: Honoraria, Membership on an entity's Board of Directors or advisory committees; Elsevier PracticeUpdate: Oncology: Consultancy, Honoraria; AbbVie/Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; Vincerx pharma: Membership on an entity's Board of Directors or advisory committees; LLC(Targeted Oncology): Consultancy; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Xcenda: Consultancy, Honoraria; Gilead: Membership on an entity's Board of Directors or advisory committees; Acerta: Consultancy, Research Funding; Rosewell Park: Consultancy; Janssen: Membership on an entity's Board of Directors or advisory committees; Elsevier's Practice Update Oncology, Intellisphere, LLC(Targeted Oncology): Consultancy; AbbVie/Pharmacyclics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; MorphoSys: Honoraria, Other; Incyte: Honoraria; Novartis: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Meyers Squibb: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genomic Testing Cooperative: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Other: Leadership role; Celgene: Consultancy, Honoraria, Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech/Hoffman la Roche: Research Funding; Janssen: Research Funding; Karyopharm: Research Funding; Michael J Hennessey Associates INC: Consultancy; Hoffman la Roche: Consultancy; Physicians' Education Resource: Consultancy, Other: Meeting/travel support; Medscape: Consultancy; Phamacyclics: Research Funding; Constellation: Research Funding; Xcenda: Consultancy; Hackensack Meridian Health, Regional Cancer Care Associates/OMI: Current Employment. Pecora: Genetic testing cooperative: Other: equity investor; Genetic testing cooperative: Membership on an entity's Board of Directors or advisory committees. Koprivnikar: Bristol Myers Squibb: Speakers Bureau. McCloskey: BMS: Honoraria, Speakers Bureau; COTA: Other: Equity Ownership; Takeda: Consultancy, Speakers Bureau; Pfizer: Consultancy; Novartis: Consultancy; Jazz: Consultancy, Speakers Bureau; Incyte: Speakers Bureau; Amgen: Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal